To a certain extent, the electrochemical performance of certain capacitors, such as EDLCs, can be enhanced by temperature. In fact, better performance can be achieved at higher temperature if all the components are well suited to this purpose. Higher temperature facilitates the migration of ions enabling them to penetrate the fine pores of the electrodes more effectively. This increases the surface area for charge storage, resulting in a higher capacitance. However, there should be a compromise between the applied temperature for optimal capacitance and the overall temperature suitable for the device’s components. Additionally, it’s very important that the device has a stable performance along the operating temperature window. [1] Capacitors are essential components in virtually every electronic system. Often, if operation temperatures lie above 125-150ºC, electronic systems need to be cooled constantly to avoid overheating and failure of individual components. Incorporating a cooling system adds complexity and cost to the overall design. Hence, the need for advancements in high temperature electronic systems for reliable and efficient active and passive components. For applications in harsh environment conditions, such as down-hole oil exploration, mining, drilling operations, and some industrial electronics, the operating temperatures can frequently exceed 175-200°C. Military electronics are built to withstand temperatures across a range from -65°C to 125°C while automotive applications may reach temperatures up to 150°C. For example, due to the proximity to engines, the ambient temperature of the inverter in HEVs can reach 140°C without an external cooling system. In other applications, such as space exploration, gas production, geothermal energy, and aerospace operating temperatures can reach up to more than 500°C. [2] Below are examples of fans and aluminium fins as cooling mechanisms in an electrical power unit from Mitsumi electric. [3] Additionally, biaxially oriented polypropylene (BOPP) films have been widely used as dielectric films in capacitors. However, the maximum operating temperature of BOPP is lower than 105°C. Additionally, the maximum operating temperature of other capacitor films such as poly(ethylene-terephthalate) (PET), polycarbonate (PC) and polystyrene (PS), etc. are also limited to 125°C. Therefore, no commercial capacitor films can meet the growing demand for high temperature (>125°C) applications, without extra cooling systems [4], adding cost and volume to the main part. On the other hand, ceramic capacitors exhibit high temperature resistance characteristics. However, ceramic materials are inherently fragile, difficult to machine with precision, and have a relatively small electrode specific surface area, resulting in low energy storage density. The current temperature range of aluminum electrolytic capacitors is limited to −50°C to 150°C, primarily restricted by the poor thermal stability of the cathode materials, such as electrolyte, MnO2, or conductive polymers. On the other hand, commercial tantalum capacitors typically withstand temperatures of around 125ºC. This limitation, alongside with the fact that tantalum raises environmental concerns due to the energy-intensive mining process and its classification as a conflict mineral (as its extraction in certain regions has been linked to human rights abuses and funding of armed conflicts) makes a lot of research aimed at replacing these components. [5] Supercapacitors are another passive component very helpful in meeting the high power requirements. However, many reports that are now focusing their research on room temperature (RT) performances may not be suitable for applications under extreme environmental temperatures, particularly wide temperature ranges. Many materials, such as activated carbons, and new types of electrolytes (such as ionic liquids) can significantly increase the temperature window of supercapacitors. Under extreme temperatures, innovations like solid-state electrolytes or hybrid capacitors help maintain efficiency. Normally supercapacitors tend to work in a temperature range of -40 to 85ºC. These new materials can help to significantly increase this window. Temperature is a critical variable that can determine the performance, life-span, and safety of energy storage devices. Achieving optimal and stable performance across a wide temperature range requires that each component of the device – electrolyte, packing case, active materials, separator – all have both thermal and electrochemical stability. The performance of the overall system is intrinsically dependent on the stability and functionality of these individual components under high-temperature conditions. Electrolyte In high-temperature applications, the properties of the electrolyte are more critical than those of electrodes or separators. In capacitors, mainly electrolytic capacitors, the electrolyte can be: The conditions under which a capacitor operates can significantly affect its performance. In aluminum electrolytic capacitors, the rise in temperature and power dissipation are directly proportional and occur when the voltage across the dielectric material varies. The efficient operation of the capacitor is highly dependent on the ambient temperature. Wide variations of the internal and external temperatures can affect its stipulated performance. High temperatures within the capacitor result in the evaporation of the electrolyte leading to its degradation. Leakage current also contributes to heat accumulation within the capacitor capsule. The electrolyte thermal stability can also significantly affect the performance of supercapacitors. Commercial supercapacitors use organic electrolytes such as tetraethylammonium tetrafluoroborate (TEABF4) dissolved in propylene carbonate (PC), or acetonitrile (ACN) which are highly flammable and harmful to the environment. [6] In high temperature environments, electrolyte can deteriorate, leading to leakage and corrosion effects. Many researchers are focused on imidazolium-based ILs as electrolytes for supercapacitors owing to their advantages, such as tunable physicochemical properties, low viscosity and high ionic conductivity. For example ethyl-methyl imidazolium tetrafluoroborate (EMIM BF4) has a wide potential window, up to 3.5 V with excellent electrochemical stability, and also exhibits a high specific capacitance for activated carbon (AC) based SCs. However, at low temperatures, the high viscosity and low electrical conductivity of imidazolium-based ILs hamper their large-scale industrial applications. Pyrrolidinum-based ILs have been widely applied in SCs and Li-ion batteries as a solid-state electrolyte. Substitution of the pyrrolidine cation improves the ionic conductivity of IL-based electrolytes. Some studies achieved a potential window of 3.5 V using a pyrrolidinium-based ionic liquid as the electrolyte. However, the high viscosity and low ionic conductivity of this electrolyte at temperatures below 50°C limited its performance. Ammonium-based ILs are also promising electrolytes due to their electrochemical stability window which is usually greater than 5 V. However, quaternary ammonium based ILs may be limited in application because of the larger size of cations, high viscosity and low ionic conductivity. New studies have mentioned an ionic liquid with a cation containing a methoxyethyl group combined with a BF4− or TFSI anion which increases the potential window and the ionic conductivity, although there is only limited research in this aspect. [7] Phosphonium-based ILs as electrolytes have not been extensively explored in large-scale applications due to their characteristically large cations and low ionic conductivities. However, this type of ILs could be an alternative electrolyte in the future as a combination with other ILs owing to their remarkable properties such as thermal stability (up to 400 °C in some cases) and fast electrochemical activity compared to imidazolium and ammonium-based ILs. There is an increased challenge in balancing the performance of electrolytes, mainly ionic liquids, with their temperature of operation window. As was previously referred to, an increasing number of applications require the samples to work at temperatures higher than 150ºC, and the electrolyte is the main factor hindering many components. Ionic liquids are a promising material for electrolytes that could open the operation window of next generation supercapacitor products. [8] Separators need to have low resistance to ion transportation, perfect wettability, strong tensile strength, and good flexibility. Additionally, they need to be chemically and electrochemically inert to all other cell components including the electrodes and electrolytes. However, commercial separators of both capacitors and supercapacitors such as polyolefin-based porous films and cellulose paper are not good choices for high-temperature applications. These separators can easily shrink at temperatures above 100ºC closing their pores, leading to electrical degradation of the capacitor. New materials in ceramic-based separators, such as Zirconia (ZrO2) and Silicon Carbide (SiC) have shown promise, offering superior thermal stability and maintaining performance at temperatures exceeding 120°C. These separators can endure high temperatures without shrinking, thus preventing short circuits and enhancing the reliability of supercapacitors in demanding applications. Another approach is aramid-based separators and mixed aramid/cellulose-based separators. Aramid nanofiber (ANF) membranes, in particular, have shown excellent performance in capacitors and supercapacitors, offering enhanced safety, reliability, and cycle life. These separators can withstand high temperatures (up to 200ºC) and maintain their structural integrity, making them ideal for advanced energy storage systems. Additionally, combining aramid with cellulose can further improve the mechanical properties and thermal resistance of the separators, providing a robust solution for high-temperature environments. Porous separators based on regenerated cellulose (RC) show higher permeability, better performance overall, and even greater thermal stability, being able to withstand temperatures higher than 200ºC. Humidity/Impurities The existence of impurities, such as chloride ions (Cl-), iron (Fe), Sulfur (S) and other metallic species, in the electrolyte and electrode materials can cause unwanted electrochemical reactions, affecting the overall performance and efficiency of capacitors and how the device behaves when exposed to extreme temperatures. In electrolytes, these impurities cause side-reactions (oxidation/reduction reactions), leading to accelerated degradation and decrease of device lifetime. The presence of water is particularly relevant for organic and IL-based electrolytes. A sample that is not correctly dried can also impact the performance of the device. When the boiling temperature of water is reached, the capacitor may suffer swelling and performance degradation. In aluminum-based capacitors, hydration of the electrode will increase the leakage current of the capacitor and reduce the capacitance. Adding a very small amount of phosphoric acid and its compounds (such as 0.05% to 5% alkyl phosphoric acid) can prevent the hydration of the oxide film. Adding silicic acid compounds can also prevent electrode hydration. An addition of 0.01%~0.5% aluminate to the ammonium borate ethylene glycol series electrolyte can help prevent deterioration of electrolyte at high temperatures. Usually the oxide film on the surface of the aluminum foil will be damaged during the opening, riveting and winding processes of the anode aluminum foil, or under storage and working conditions. While the electrolyte repairs these dielectric films, hydrogen gas will be produced at the negative electrode, which will increase the internal pressure of the capacitor, shortening the service life of the capacitor, and even risking bursting at high temperatures. In aluminum-based current collectors, it’s crucial to be aware of halide impurities like chloride ions Cl–. These ions can severely damage the electrode structure, causing anode corrosion and lowering the rated voltage. Additionally, heavy metal ions such as copper Cu2+ and iron Fe3+ can form micro galvanic cells on the anode aluminum foil, leading to its corrosion. This process significantly increases the leakage current of the product and diminishes its overall reliability. [6] Conclusion Temperature is a crucial factor for the performance of capacitors and supercapacitors. Although some capacitors’ performance can be improved at room temperature and slightly above, a continuous operation near or above maximum working temperature will most likely result in a loss of properties, and in many instances can lead to catastrophic failures. This presents a huge issue, especially when taking into consideration that an increasingly larger number of applications require that the passive components work at high temperatures. From hybrid vehicles to aerospace and mining applications, passive components such as capacitors, or supercapacitors are key to the performance of several devices. Not only are they essential components, but they are also one of the main sources of component failure. This means that improving the performance and durability of these components, not only but mainly at high temperature and harsher conditions is a constant and growing necessity. Electrolyte and separators are presented as the main sources of capacitor and supercapacitor failure at high temperatures. These components present a challenge due to the growing need to adapt to increasingly high temperature, while maintaining overall performance in the device. Other factors, such as impurities and humidity can contribute to a decrease in lifetime performance, and can be affected by temperature. [2] Alshatnawi, F., Enakerakpo, E. Alhendi, M. et al, “High stability and reliability additively manufactured metal-insulator-metal capacitors for high-temperature applications,” Materials Today Communications, vol. 39, pp. 108682, June 2024, doi: 10.1016/j.mtcomm.2024.108682. [3] © Raimond Spekking / CC BY-SA 4.0 (via Wikimedia Commons). Available: https://commons.wikimedia.org/wiki/File:Mitsumi_Electric_SR68-05550M-7350.jpg [4] Liu, X-J., Zheng, M-S., Chen, G., et al, “High-temperature polyimide dielectric materials for energy storage: theory, design, preparation and properties,” Energy Environ. Sci., vol. 15, pp. 56-81, 2022, doi: 10.1039/D1EE03186D. [5] Guo, Y., Wang, S., Du, X., et al, “High-performance MIM-type aluminum electrolytic capacitors with durable waterproof and wide temperature window,” Energy Storage Materials, vol. 71, August 2024, pp. 103685, doi: 10.1016/j.ensm.2024.103685. [6] Xuansn, “Electrolyte of Aluminum Electrolytic Capacitor.” Available: https://www.xuanxcapacitors.com/electrolyte-of-aluminum-electrolytic-capacitor.html/ [7] Sato, T., Masuda, G., and Takagi, K., “Electrochemical properties of novel ionic liquids for electric double layer capacitor applications,” Electrochimica Acta, vol. 49, 21, pp. 3603-3611, September 2004, doi: 10.1016/j.electacta.2004.03.030. [8] Abdelhafiz, M., Al-Rubaie, K. S., Emadi, A., and Elbestawi, M. A. (2021). “Process–Structure–Property Relationships of Copper Parts Manufactured by Laser Powder Bed Fusion,” Materials, vol. 14, 11, pp. 2945, doi: 10.3390/ma14112945. [9] Fan, L.-L., Li, H.-J., and Chen, Q.-H., “Applications and Mechanisms of Ionic Liquids in Whole-Cell Biotransformation. International Journal of Molecular Sciences,” vol. 15, 2014, pp. 12196-12216, doi: 10.3390/ijms150712196. High-Temperature Effects on Capacitor Performance: A Focus on Component Stability

Recently there has been an increasing need for electronic components, such as capacitors and supercapacitors, to work in harsh environments. From industrial machinery to aerospace and renewable energy systems, electronic systems are now deployed in applications where they must endure extreme conditions, such as high temperatures, elevated pressures, and severe mechanical stresses. This presents significant technical challenges, requiring advanced designs and materials to ensure that components can maintain performance and reliability under such conditions. The operating temperatures of electrochemical energy storage devices are mainly limited by the thermophysical properties and stability of their components, particularly electrode and electrolyte degradation and separator instability at higher temperatures. Capacitance and equivalent series resistance are not fixed values. They change with temperature, and are two important electrochemical performance metrics of all types of capacitors and supercapacitors dictating their energy and power densities. Other important metrics are aging, self-discharge and leakage.
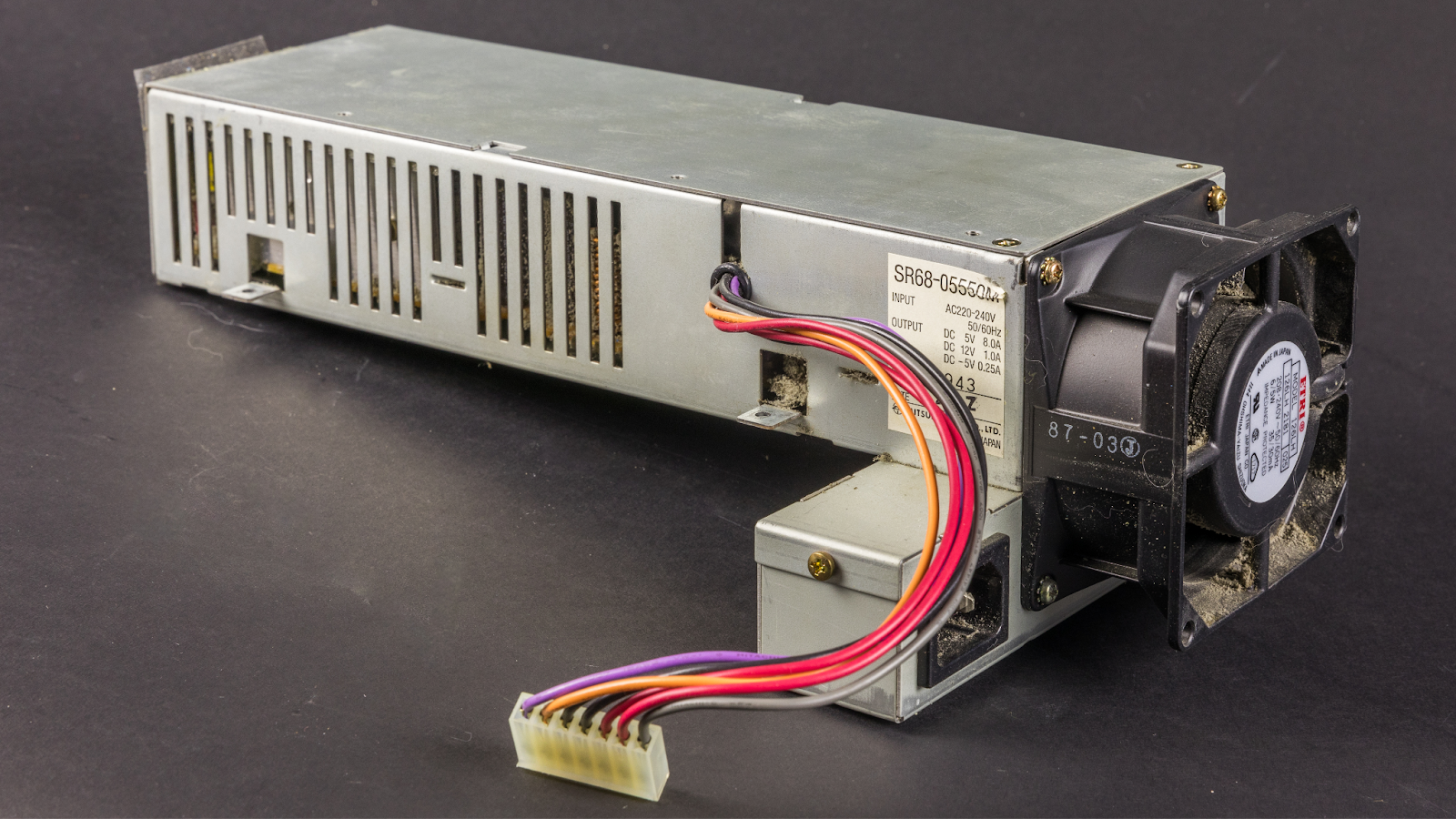
High temperature capacitor applications
Capacitors have long been recognized as a challenging passive component for usage in high temperature environments. Polymer film capacitors have numerous applications such as hybrid electric vehicles (HEVs), grid-connected photovoltaic systems, wind power generation, and underground oil and gas exploration. However, the development of the above-mentioned fields poses greater challenges to the heat resistance of these film capacitors. [2]
Influence of temperature on life-time performance
Separators
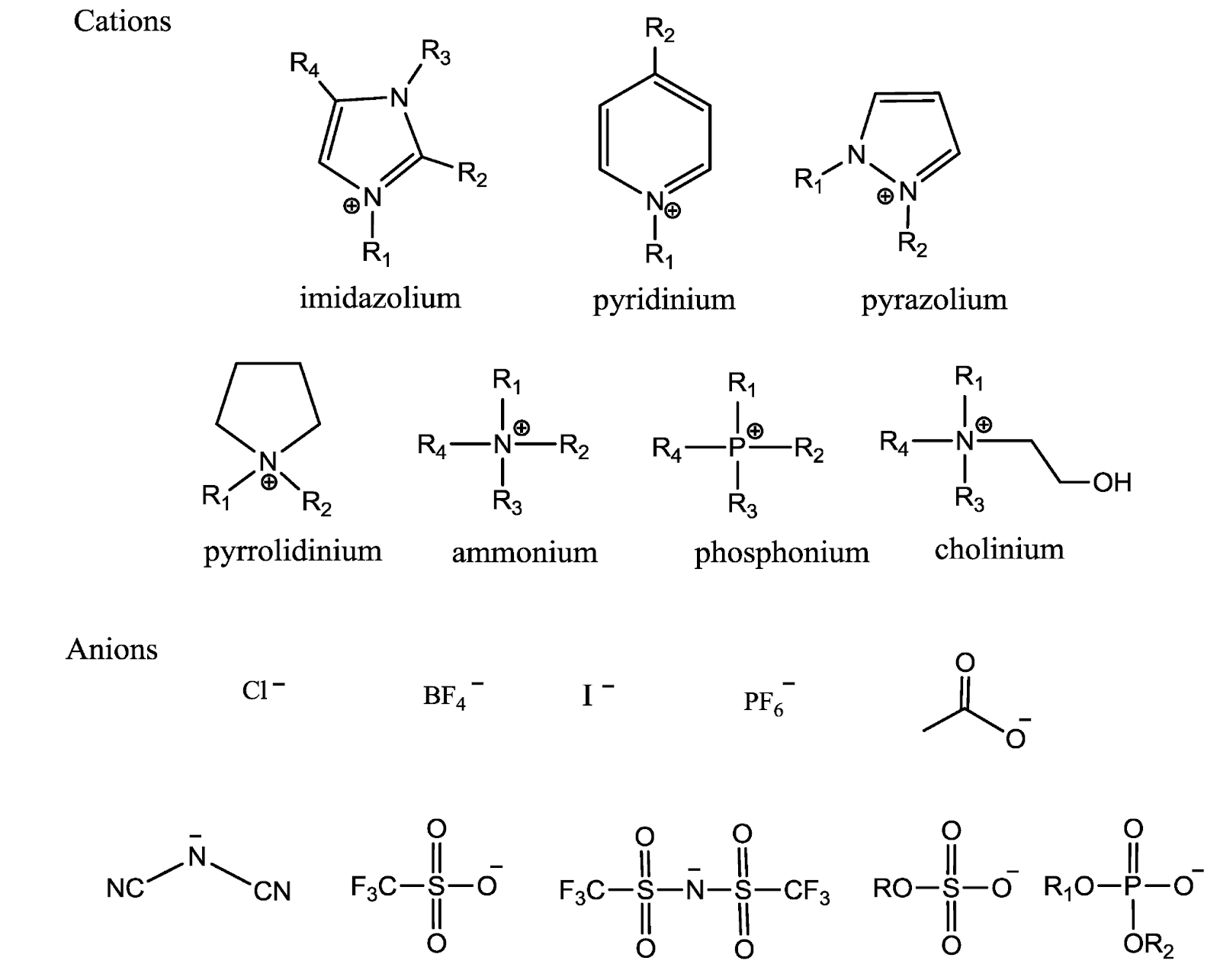
In the P.CAP project we are working at the forefront of supercapacitor materials to improve the frequency response and high temperature tolerance of our devices. We expect to have working prototypes by late 2025. Stay connected to learn more, and if you found this white paper valuable, feel free to share your thoughts in the form below.
[1] Borges, R., Reddy, A., Rodrigues, MT. et al, “Supercapacitor Operating At 200 Degrees Celsius,” Sci Rep 3, 2572, September 2013, doi: 10.1038/srep02572.
This website uses cookies to improve your web experience.


